Pharmaceutical clinical trials: The perfect catalyst for digital transformation?
Clinical trials can be sped up by pairing data-driven modernisation of systems and processes with AI-enabled tools
The outcome of pharmaceutical clinical trials (the release of new drugs to the market) has a massive human and financial value — the quicker trials can be completed, the faster companies can get medication and treatments to patients. However, this highly regulated and documentation-heavy process can be extremely slow. Data-driven modernisation of systems and processes, paired with AI-enabled tools, can speed up clinical trials without compromising on safety or compliance to regulations.
Sources:
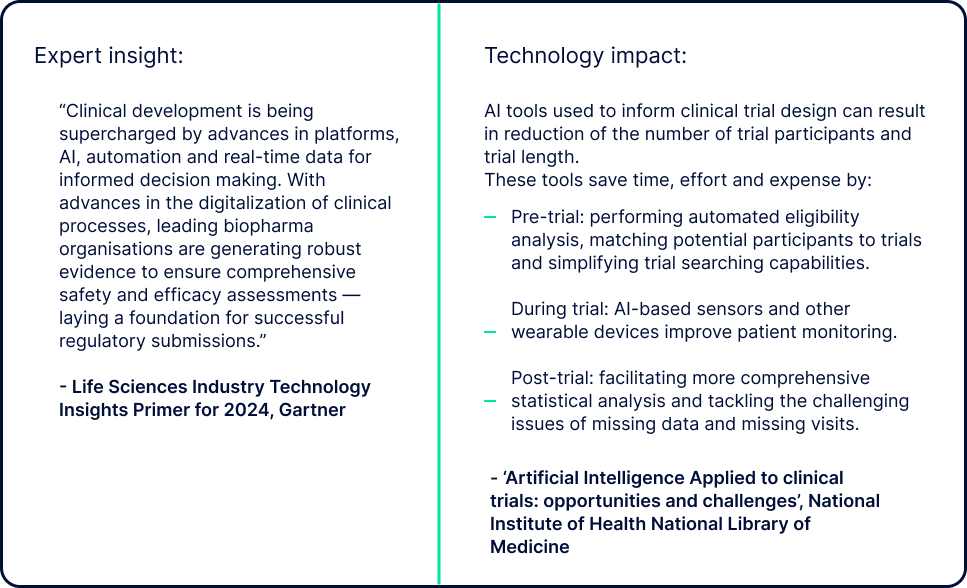
Gartner, 'Life Sciences Industry Technology Insights Primer for 2024’, By Healthcare and Life Sciences Research Team, 31 January 2024. GARTNER is a registered trademark and service mark of Gartner, Inc. and/or its affiliates in the U.S. and internationally and is used herein with permission. All rights reserved.
'Artificial Intelligence Applied to clinical trials: opportunities and challenges', National Institute of Health National Library of Medicine.
"AI transformation" vs "status quo": Faster analysis of clinical trial data
One of the primary reasons that clinical trials take as long as they do is data. The way that data is captured, managed and analysed can have monumental effects on the speed with which results are obtained.
Factors that slow down the trial process include: fragmented data/disconnected systems, repetitive/duplicative manual processes such as data transcription from disparate sources and “reinventing the wheel” by creating new databases for each new study.
Integrating AI technology into the capture and analysis of clinical trial datasets along with cloud-based storage and Open Source software can dramatically accelerate the data-gathering process.
When data from a variety of sources is more easily accessed and shared among different stakeholders, it can be standardised and structured by AI tools. These tools can also help to automate data management, auto-populate required documentation and analyses, and even review the results of other studies to generate insights and suggest efficiencies.
For example, Insilico Medicine completed a preclinical development from hypothesis to preclinical drug candidate in under 18 months at a cost of only $2.6 million. This is several orders of magnitude faster and cheaper than traditional drug discovery approaches.
Meeting regulatory requirements while accelerating timelines with digital services
Leaders in the pharmaceutical industry are understandably hesitant to modernise their operations because of the risk associated with the (perceived) possibility of becoming non-compliant with numerous complex regulations. Existing processes are extremely important, because they have been developed to safeguard trial participants and ensure clear documentation of all procedures and results.
It’s important to note that data and AI solutions implemented for rapid modernisation don’t seek to eliminate or circumvent any established rules. Instead, cloud-based, Open Source software and AI-enabled tools can gather, categorise and streamline data, and reduce the amount of duplicate work done to comply with all existing regulatory requirements.
Modernised tools, coupled with cultural shifts, can expedite drug development
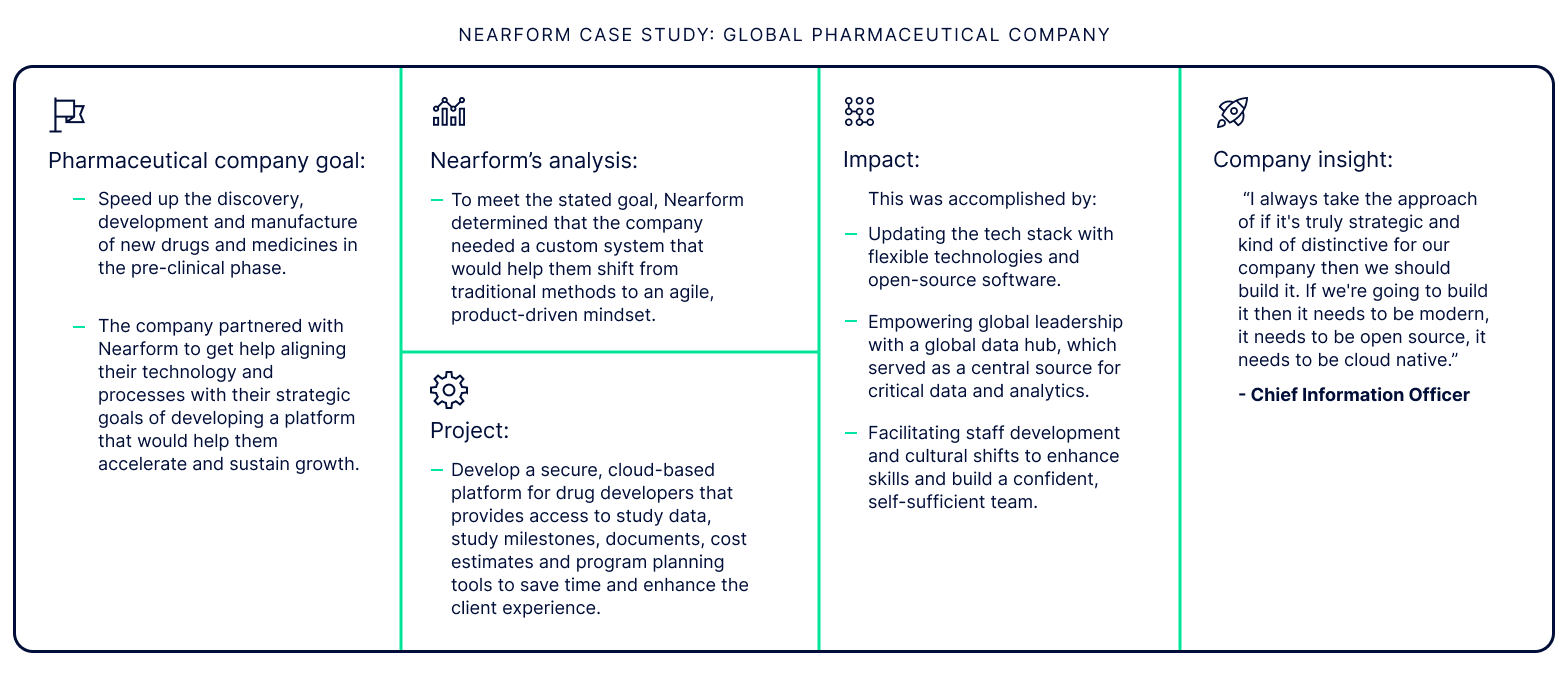
Implementing new data-driven modernisation strategies is so much more than simply “digitising” existing processes. It involves a deep understanding of the existing workflow to conceptualise how it can be reimagined more efficiently. Transitioning company culture to an agile mindset is key as well, because often existing procedures have been designed with short-term goals in mind, limiting the ability to address long-term goals. Agility also encourages greater adaptability, and a culture of continuous improvement.
When consultants and engineers partner and collaborate to share their institutional knowledge and experience with each other, the results can be transformative. Together, they can bridge previously siloed data, enhance efficiencies, drive positive staff and patient experiences and prepare the company for sustainable growth.
Insight, imagination and expertly engineered solutions to accelerate and sustain progress.
Contact

